I first tested the XRF on some known metals just to confirm its accuracy and repeatability, and it was very accurate and very repeatable. I tested stainless steel, aluminum, and carbon steel.
It should be noted that this test doesn't date the metal, however it should show if the steel is a modern day iron or bloomery iron. It's certainly possible someone could make their own iron and then process it down and forge it into a sword and then artificially age it, however as a bladesmith myself, I would find that very hard to believe. Making your own steel out of dirt is a major undertaking and then trying to forge that bloom into a bar and then a sword would be tough. A faker would buy bar stock and start the forging process from there.
The first picture shows the four swords.
The two on the left are the meteorite swords I forged. They were both major projects and took months to complete. Forging meteorite is not easy and it actually took a couple of years to get the process down. They are 100% meteorite iron. The XRF results back it up.
The third sword is a Viking Age sword I bought from an antique arms dealer last year. I have always been very confident in it's authenticity. The XRF results also back this up in my opinion. A modern day iron would not have Sulfur and Phosphorus levels this high. Also there is pretty much zero Manganese, a modern day iron would have much higher Manganese. The Silicon levels seem high too. In addition the composition varies as you measure up and down the blade which would probably not happen with a modern day iron.
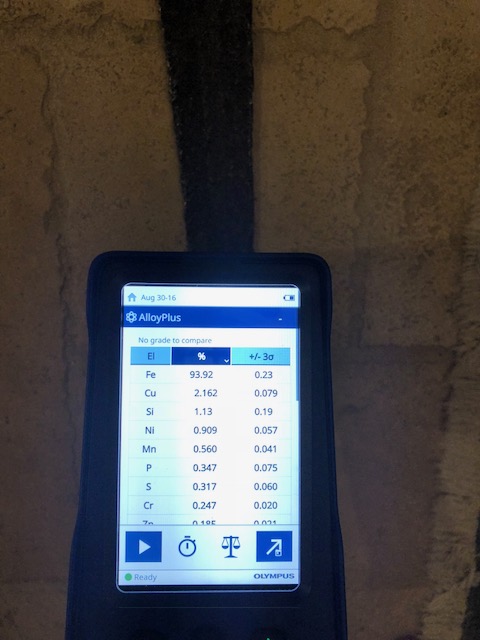
The fourth sword is a Medieval Age sword I recently bought on Ebay. I knew I was taking a big chance with it. When I got it it sure seemed real to me, but then I found a thread in this forum with other swords sold on Catawiki that appeared to be very similar to mine and the consensus on that thread was that they are modern day fakes. That made me think mine is probably fake because they seem so similar. The XRF results were very surprising in my opinion. The Sulfur and Phosphorus levels seem too high to be a modern day iron. The Silicon levels seem very high too. The most surprising thing in my opinion is it has 2% - 3% copper depending on where it was analyzed. I've never heard of steel with that much copper. I just don't see how this could be a modern day iron alloy. That being said I'm not a metallurgist.
I'd love to get your opinion on these results - especially with the Medieval Age sword. Thanks!!!
[ Download ]
[ Download ]
[ Download ]
[ Download ]