After looking at many different styles of swords that had complex etching on the blades, the thought occurred to me that, while etching on a blade certainly makes it attractive, is it something you'd want on a sword intended for use?
I ask this because it seems to me that if you used an etched blade in a fight, and the blade was marked with blood, the blood would be near impossible to completely remove from the etched patterns or pictures. It would stand to reason then that you would have a terrible case of rust spots on your very attractive, etched blade.
It also seems to me that etching would weaken the structural endurance of the blade.
Any thoughts on this?
| Nathan Gilleland wrote: |
| After looking at many different styles of swords that had complex etching on the blades, the thought occurred to me that, while etching on a blade certainly makes it attractive, is it something you'd want on a sword intended for use?
I ask this because it seems to me that if you used an etched blade in a fight, and the blade was marked with blood, the blood would be near impossible to completely remove from the etched patterns or pictures. It would stand to reason then that you would have a terrible case of rust spots on your very attractive, etched blade. It also seems to me that etching would weaken the structural endurance of the blade. Any thoughts on this? |
Blood shouldn't be a problem. Might be difficult to remove in the field, but fine, stiff brushes and solvents(even water) would remove everything fine.
And the acid etching only eats away a shallow bit of surface. Unless you leave the acid on the blade for far too long, the blade will remain perfectly functional.
I ran into this question too. I want to make some etching on the blade and pommel. I will use photo-etching, for I' have some experience in making circuit boards.
Now my greatest concern is finding a mordant. For circuit boards I use ferric-chloride. It works fine for copper because it's more chemically active than iron, some say it might work for stainless steel too due to it's composition, but will certainly not work on iron or carbon steel.
Acids are hard to procure, especially nitric acid which is blacklisted (for other obvious reasons). I know there are special mordants on the market, but not in my country. Buying and shipping will be problematic too.
Another way is electrochemical etching. I have the necessary hardware for this. I'm doing electrolythical rust cleaning for some time on my old rusty bayonets. For etching you have just to reverse the polarity but there are other issues too. I have to protect the entire surface of the piece (let's say a pommel) except the parts that needs etching and... now how do I connect the wire? I could try soldering and protecting it with wax or laquer but it's easier said than done especially when different sides of the piece must be exposed to UV light for the method to work.
Please advice.
Now my greatest concern is finding a mordant. For circuit boards I use ferric-chloride. It works fine for copper because it's more chemically active than iron, some say it might work for stainless steel too due to it's composition, but will certainly not work on iron or carbon steel.
Acids are hard to procure, especially nitric acid which is blacklisted (for other obvious reasons). I know there are special mordants on the market, but not in my country. Buying and shipping will be problematic too.
Another way is electrochemical etching. I have the necessary hardware for this. I'm doing electrolythical rust cleaning for some time on my old rusty bayonets. For etching you have just to reverse the polarity but there are other issues too. I have to protect the entire surface of the piece (let's say a pommel) except the parts that needs etching and... now how do I connect the wire? I could try soldering and protecting it with wax or laquer but it's easier said than done especially when different sides of the piece must be exposed to UV light for the method to work.
Please advice.
Lemon juice has a lot of citric acid and it will etch but it is fairly weak acid so it won't work fast or cut deeply but it does work to a degree.
Better for creating surface texture or aging a surface than a strong acid that can cut precise lines.
Not sure if one could use a large quantity of lemon juice and boil it down to increase the concentration/strength of the acid.
http://en.wikipedia.org/wiki/Citric_acid
Concentrating it to 2X or 3X it's natural strength should still be safe but I think one should know a lot more about the subject before trying for higher concentrations that might be dangerous in causing acid burns or other hazards.
Better for creating surface texture or aging a surface than a strong acid that can cut precise lines.
Not sure if one could use a large quantity of lemon juice and boil it down to increase the concentration/strength of the acid.
http://en.wikipedia.org/wiki/Citric_acid
Concentrating it to 2X or 3X it's natural strength should still be safe but I think one should know a lot more about the subject before trying for higher concentrations that might be dangerous in causing acid burns or other hazards.
Many etchants actually help protect a blade from rust. it is not as good a protection as a gun blueing, but I have found that in my shop, especially in the spring where every morning I have a thin orange layer on my tools, that any blades I make must be oiled or waxed or they will rust before I can finish them. The pattern-welded ones can usually resist rusting for a few days.
Actually Ferric-Chloride works very well on steels. It is the preferred etchant for most damascus makers. Another etchant that works well and is easy to get is pH minus or pH down. it is a salt that can be added to pools to lower their pH. It works by creating a sulfuric acid. pH down is avaliable at most stored that sell pool chemicals, and some outdoor/yard stores. It comes as crystals. I generally mix 1 container with 5 gallons of water.
If you are interested in trying Citric acid there are places to find it. Citric acid is a very active acid when it comes to steels. We use it in the pharmaceutical and food and beverage industries to clean the stainless steel lines to make sure they do not corrode. The citric acid will dissolve all of the free iron and impurities. Citric acid can be bought in gallon or larger containers form many stores that serve restaurants and beverage companies. It is also available in cup to pint sized containers at many stores that sell dishwashers, and many department stores. Look for washing machine cleaners. most are concentrated citric acid.
For your pommel, is it threaded or drilled all the way through? if it is threaded, you could screw in a bolt before you cover it with mordant, if it is a pass through, you could use a wire through the pommel, bent in a zig-zag so that you have a good contact, and then bent at the bottom to hold the pommel on the wire.
I have also found a recipe that was used several centuries ago for etching. 2 parts table salt, 1 part copper sulfate, and enough water to make a paste. It takes a bit longer than the other chemicals, but leaves a nice dark etch.
Actually Ferric-Chloride works very well on steels. It is the preferred etchant for most damascus makers. Another etchant that works well and is easy to get is pH minus or pH down. it is a salt that can be added to pools to lower their pH. It works by creating a sulfuric acid. pH down is avaliable at most stored that sell pool chemicals, and some outdoor/yard stores. It comes as crystals. I generally mix 1 container with 5 gallons of water.
If you are interested in trying Citric acid there are places to find it. Citric acid is a very active acid when it comes to steels. We use it in the pharmaceutical and food and beverage industries to clean the stainless steel lines to make sure they do not corrode. The citric acid will dissolve all of the free iron and impurities. Citric acid can be bought in gallon or larger containers form many stores that serve restaurants and beverage companies. It is also available in cup to pint sized containers at many stores that sell dishwashers, and many department stores. Look for washing machine cleaners. most are concentrated citric acid.
For your pommel, is it threaded or drilled all the way through? if it is threaded, you could screw in a bolt before you cover it with mordant, if it is a pass through, you could use a wire through the pommel, bent in a zig-zag so that you have a good contact, and then bent at the bottom to hold the pommel on the wire.
I have also found a recipe that was used several centuries ago for etching. 2 parts table salt, 1 part copper sulfate, and enough water to make a paste. It takes a bit longer than the other chemicals, but leaves a nice dark etch.
| Ken Nelson wrote: |
|
Actually Ferric-Chloride works very well on steels. It is the preferred etchant for most damascus makers. Another etchant that works well and is easy to get is pH minus or pH down. |
Ferric Chloride does work really well. I know a couple of knife makers who have used in the past it to etch their maker's mark. But, I think the electrochemical etchants for maker's marks are actually something else.
A nice tutorial on modern etching from John Lundemo here
http://www.swordforum.com/forums/showthread.php?t=19927
As to care and feeding of new blades (or new in the period use) ordinary care requires only ordinary care aside from abrasives which will erode the relief of the design.
Pin etching of the fine blue and gold is not much more complicated, as the blade was covered with a resist and then the design drawn. That effect looks at times like fine line engraving but is indeed just a resist and removing what the design will reveal.The fire gilding and blue a bit more complicated and yes, those blades did end up losing a lot of the finish in serious use. These generally extraordinarily elaborate swords were usually reserved for dress and parade, as well presentations.
Cheers
GC
http://www.swordforum.com/forums/showthread.php?t=19927
As to care and feeding of new blades (or new in the period use) ordinary care requires only ordinary care aside from abrasives which will erode the relief of the design.
Pin etching of the fine blue and gold is not much more complicated, as the blade was covered with a resist and then the design drawn. That effect looks at times like fine line engraving but is indeed just a resist and removing what the design will reveal.The fire gilding and blue a bit more complicated and yes, those blades did end up losing a lot of the finish in serious use. These generally extraordinarily elaborate swords were usually reserved for dress and parade, as well presentations.
Cheers
GC
Thank you for your advices. Finally I got some 52% nitric acid from a galvanizing workshop. I tried the photo method that I use for making circuit boards on scrap metal piece, but it wasn't resistent enough for nitric acid. Too bad I can't find the meterial described in the forum. I can try wax or asfalt combined with photo lacquer to give me the outlines for the design. I want a deeper etching and I'm wondering about what mordant they use at Albion when etching the logo on the blade (http://www.youtube.com/watch?v=cTg0Oc0mQy4).
First I want to etch with something like this on the flat part of the pommel (30 mm in diameter):
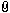 Attachment: 43.09 KB
Attachment: 43.09 KB

First I want to etch with something like this on the flat part of the pommel (30 mm in diameter):

I haven't done any etching in a few decades but in art school I took a course in it, so maybe art supply stores that sell copper sheets for etching would have the right acid and the resist.
If I remember correctly it as some form of lacquer that one brushed on the whole surface of the plate and used a sharp needle to draw/scratch in the design.
One would submerge the plate in a shallow pan of acid and brush away air bubbles away so that the acid would cut clean and crisp lines. It was important to remove the plate and check on the work the acid had done and put the plate back into the acid to cut deeper into the plate.
One might also check for spots where the resist seemed to be thinning and reapply/touch up the resist where needed.
So I would look at art supply stores that sell supplies for art etching. ( I assume that one could go to an art school and ask them where they get their supplies if one can't find a supplies source ? ).
If I remember correctly it as some form of lacquer that one brushed on the whole surface of the plate and used a sharp needle to draw/scratch in the design.
One would submerge the plate in a shallow pan of acid and brush away air bubbles away so that the acid would cut clean and crisp lines. It was important to remove the plate and check on the work the acid had done and put the plate back into the acid to cut deeper into the plate.
One might also check for spots where the resist seemed to be thinning and reapply/touch up the resist where needed.
So I would look at art supply stores that sell supplies for art etching. ( I assume that one could go to an art school and ask them where they get their supplies if one can't find a supplies source ? ).
| Ozsváth Árpád-István wrote: |
| I tried the photo method that I use for making circuit boards on scrap metal piece, but it wasn't resistent enough for nitric acid. Too bad I can't find the meterial described in the forum. |
Was your photo transfer method similar to this? http://www.ganoksin.com/borisat/nenam/photocopy_transfer_etch.htm
No I use another method for making circuit boards:
The cleaned surface is sprayed with photo-lacquer (POSITIV 20). After I print the design on transparency I place it over the surface and put it under UV light for 3-5 minutes. A 0.7% NaOh solution will remove the lacquer from the parts exposed to UV light leaving only the covered parts with a thin plastic film layer.
Now I put the piece in 40% FeCl solution that will slowly eat away the copper following the rules of chemistry:
FeCl3 + Cu -> FeCl2 + CuCl
FeCl3 + CuCl -> FeCl2 + CuCl2
It works like wonder for thin copper plates like circuit boards, but my pommel is iron (well, a very low grade steel) and iron won't react with ferric-chloride.
Nitric acid is a more aggressive chemical that will react with iron, but seems to corrode photo-lacquer and I'm pretty sure it will destroy toner paint too.
Some recomanded shellac or asfalt, materials that resist to acids, but it's all over for the photo transfer.
The cleaned surface is sprayed with photo-lacquer (POSITIV 20). After I print the design on transparency I place it over the surface and put it under UV light for 3-5 minutes. A 0.7% NaOh solution will remove the lacquer from the parts exposed to UV light leaving only the covered parts with a thin plastic film layer.
Now I put the piece in 40% FeCl solution that will slowly eat away the copper following the rules of chemistry:
FeCl3 + Cu -> FeCl2 + CuCl
FeCl3 + CuCl -> FeCl2 + CuCl2
It works like wonder for thin copper plates like circuit boards, but my pommel is iron (well, a very low grade steel) and iron won't react with ferric-chloride.
Nitric acid is a more aggressive chemical that will react with iron, but seems to corrode photo-lacquer and I'm pretty sure it will destroy toner paint too.
Some recomanded shellac or asfalt, materials that resist to acids, but it's all over for the photo transfer.
At one time Albion was using ferric chloride for some etching, I know that several other smiths who use it, and I have used it in the past. Here is one blade that I etched using it. I have also used it to etch pattern welding and to bring out hamon.
[ Linked Image ]
Shane
[ Linked Image ]
Shane
| Ozsváth Árpád-István wrote: |
|
Some recomanded shellac or asfalt, materials that resist to acids, but it's all over for the photo transfer. |
Yeah, the engraving I did back then was hand drawn so not a photographic process so the resist was probably some lacquer or shellac. I think it was an amber liquid ? But this was a long time ago.
I would research 19th century and earlier traditional engraving techniques, art schools or art supply stores for information as I suggested before if you could hand engrave your design. ( Not directly applicable for a photographic process but might still give some interesting options about acids and resists ..... although probably for copper plate and not steel ).
http://en.wikipedia.org/wiki/Etching
Deep etching, scroll down to info about acids to use and concentrations:
http://chestofbooks.com/crafts/machinery/Rece...luids.html
Page 1 of 1
You cannot post new topics in this forumYou cannot reply to topics in this forum
You cannot edit your posts in this forum
You cannot delete your posts in this forum
You cannot vote in polls in this forum
You cannot attach files in this forum
You can download files in this forum
All contents © Copyright 2003-2006 myArmoury.com — All rights reserved
Discussion forums powered by phpBB © The phpBB Group
Switch to the Full-featured Version of the forum
Discussion forums powered by phpBB © The phpBB Group
Switch to the Full-featured Version of the forum