Hello to everyone
I'm sorry that my first post has to be a stupid question, but for me, It's very important to know.
I'm currently visiting a blade-forging- course , and during forging a large blade, i forgot a little about the time, and when I removed the raw blade from the fire, it was sparkling, like if the steel would be burning away (but just a little bit, not like in the extreme cases). When the steel cooled down, no one in the course was able to see any damage in the steel like pits and stuff.... I asked the course instructor, if I had to be concerned about what happened, or if could still use the steel for a good blade, and he wasn't able to answer me....
What do you think? Did the steel use some of its quality? Did it suffer?
I would be able to start a new blade next week, without having wasted a lot of time/work, and I would certainly do it, because I want to be the blade as perfect as possible.
I hope you do understand my problem, even if my english is so bad........
Aurelia
Greetings, and congratulations for trying your hand at forging.
to answer your question, YES, you did damage the steel, some of it may be recoverable, some of it not.
What will not be recoverable, is the amount of carbon lost from the blade. All forging will cause a little carbon loss, but careful control of temperature, and speed can keep it to a minimum that is easily ground away when it comes time to do the cold work part of the blade. Ok, perhaps it may be recoverable, but it will take a system of carburizing without access to air, something that cannot be done accurately in a forge, will take several hours, and a high level of skill and understanding to try to get close to what you were provided with by the steel industry.
The other problem you are going to run across is grain growth. Large grains can weaken the steel. It will be more brittle at a given hardness. This part of the problem is easier to deal with. Heat the steel to critical temperature and let it air cool several times, this is known as normalizing. Critical temperature is the temperature where the crystal structure in the steel changes. Ask to see a spec sheet on the steel you are using. the normalizing temperature should be on there. if not, use the austinizing temperature for hardening. Many people may say that you can use a magnet, and when the magnet no longer sticks, you have the right temperature, but depending on your steel, that could be 100 deg C off. A magnet is just a rough guess.
What steel are you using, and what kind of blade are you making? That would help to determine what temperatures, and how much the steel may have changed while burning.
Ken
to answer your question, YES, you did damage the steel, some of it may be recoverable, some of it not.
What will not be recoverable, is the amount of carbon lost from the blade. All forging will cause a little carbon loss, but careful control of temperature, and speed can keep it to a minimum that is easily ground away when it comes time to do the cold work part of the blade. Ok, perhaps it may be recoverable, but it will take a system of carburizing without access to air, something that cannot be done accurately in a forge, will take several hours, and a high level of skill and understanding to try to get close to what you were provided with by the steel industry.
The other problem you are going to run across is grain growth. Large grains can weaken the steel. It will be more brittle at a given hardness. This part of the problem is easier to deal with. Heat the steel to critical temperature and let it air cool several times, this is known as normalizing. Critical temperature is the temperature where the crystal structure in the steel changes. Ask to see a spec sheet on the steel you are using. the normalizing temperature should be on there. if not, use the austinizing temperature for hardening. Many people may say that you can use a magnet, and when the magnet no longer sticks, you have the right temperature, but depending on your steel, that could be 100 deg C off. A magnet is just a rough guess.
What steel are you using, and what kind of blade are you making? That would help to determine what temperatures, and how much the steel may have changed while burning.
Ken
Ken's answer is right.
I also "burned" the steel my first time using a coal fire to make a simple spoon for applying flux in later pattern welding projects. (I neglected it for a while with an electric fan that was evidently running a little too high.) The section of the steel sparking in the direct heat may turn mostly into iron oxide, depending upon duration, and can become so brittle that it breaks under subsequent hammer blows. That is pretty much "garbage" if it has happened. A less severe case may only involve loss of carbon, but still have enough intact iron that forging and shaping can be completed. The later case generally means that it will not be as capable of hardening when you go to quench it. I have only done this a couple of times, but the "brittle when struck by hammer" as well as long length of time it continued to spark after I pulled it from the coal (lets say between 30 seconds and 1 minute) seemed to be indicators that it was ruined as far as "good blade" purposes.
I also "burned" the steel my first time using a coal fire to make a simple spoon for applying flux in later pattern welding projects. (I neglected it for a while with an electric fan that was evidently running a little too high.) The section of the steel sparking in the direct heat may turn mostly into iron oxide, depending upon duration, and can become so brittle that it breaks under subsequent hammer blows. That is pretty much "garbage" if it has happened. A less severe case may only involve loss of carbon, but still have enough intact iron that forging and shaping can be completed. The later case generally means that it will not be as capable of hardening when you go to quench it. I have only done this a couple of times, but the "brittle when struck by hammer" as well as long length of time it continued to spark after I pulled it from the coal (lets say between 30 seconds and 1 minute) seemed to be indicators that it was ruined as far as "good blade" purposes.
If you're forging on coals/charcoal, and you see sparks when you get the metal out, immediately put it back in the fire, but in a cooler part of the fire until it stops sparking. That does two things: it stops burning, and it stops carbon loss. As it's in a reducing environment, it may actually take up carbon. Chances are that it has already taken up a lot of carbon while you were heating it. There may actually be a greater chance that you'll have too much carbon in it then too little, making it red short and crumble when you forge it. A sax I made, which as a steel edge welded to wrought iron was overheated a lot during the welding process, as I didn't know what I was doing. I think that at least 1/3rd of the steel had burned away. Nevertheless it's got one of the finest edges on it on any blade I've made so far, sharpening up to shaving sharp with ease.
But at any rate, don't burn your steel, it's not recommended ;)
But at any rate, don't burn your steel, it's not recommended ;)
I am sorry, but steel does not attain carbon that way. Even in a reducing fire in a forge, the steel is getting hit with a lot of CO2. both O2 and CO2 will pull carbon from the steel, not to mention that if you had the piece in the fire for 5 minutes, the layer of scale that will form as soon as you bring it out of the fire is just as thick, thereby removing any possible added carbon.
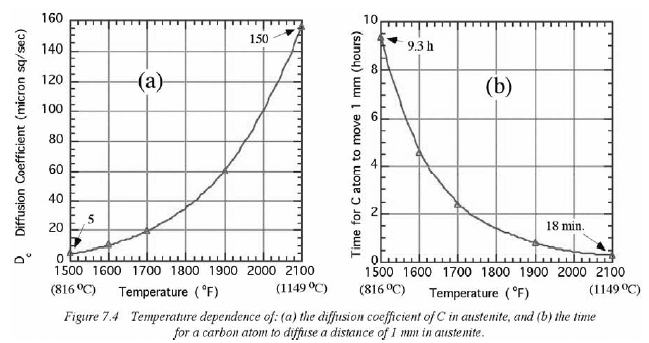
I know that "only a coal/charcoal fire can add carbon" is a long standing blacksmith tradition, but it has been tested and found to be a myth. Several hundred samples were treated in forges, and then cut apart and inspected with a microscope. they were all found to have varying levels of carbon loss on the edges. Some were more severe than others, but to add carbon to steel, you need to seal it in an environment without oxygen. that means either using a case carburizing compound which seals the surface or to pack it in carbon containing material, seal it in a tube and then roast the tube for several hours. Carbon only moves through steel at a rate measured in microns per minute. Scale forms thicker and quicker.
I am glad to hear that your seax turned out well, welding an edge steel on is much more difficult than just forging out a straight bar. What steel did you use for the edge? If you used something like W1 or O6 with well over 1.00% carbon, you could lose quite a bit and still get a fully hardenable steel. 1095 is good for that too.
(edited to add)
I did run across a process of using a forge to add carbon. but it is not nearly as simple as doing anything during forging. In Yoshindo Yoshira's book, he adds carbon to the tamahagane by building up a large charcoal fire, putting the steel near the top, and then adding a bit more charcoal. he then starts the fire, and gets the steel to temperature and then shuts off the blast, and lets the fire slowly burn overnight. I haven't seen what levels of carbon have been added, but He has done a lot of research as well, even to the point of being able to tell how much carbon is lost through each weld in the forming of the blade steel. If I remember correctly, his tamahagane starts at about 1.3% carbon, and ends up after several folds with a blade around .75% carbon.
I know that "only a coal/charcoal fire can add carbon" is a long standing blacksmith tradition, but it has been tested and found to be a myth. Several hundred samples were treated in forges, and then cut apart and inspected with a microscope. they were all found to have varying levels of carbon loss on the edges. Some were more severe than others, but to add carbon to steel, you need to seal it in an environment without oxygen. that means either using a case carburizing compound which seals the surface or to pack it in carbon containing material, seal it in a tube and then roast the tube for several hours. Carbon only moves through steel at a rate measured in microns per minute. Scale forms thicker and quicker.
I am glad to hear that your seax turned out well, welding an edge steel on is much more difficult than just forging out a straight bar. What steel did you use for the edge? If you used something like W1 or O6 with well over 1.00% carbon, you could lose quite a bit and still get a fully hardenable steel. 1095 is good for that too.
(edited to add)
I did run across a process of using a forge to add carbon. but it is not nearly as simple as doing anything during forging. In Yoshindo Yoshira's book, he adds carbon to the tamahagane by building up a large charcoal fire, putting the steel near the top, and then adding a bit more charcoal. he then starts the fire, and gets the steel to temperature and then shuts off the blast, and lets the fire slowly burn overnight. I haven't seen what levels of carbon have been added, but He has done a lot of research as well, even to the point of being able to tell how much carbon is lost through each weld in the forming of the blade steel. If I remember correctly, his tamahagane starts at about 1.3% carbon, and ends up after several folds with a blade around .75% carbon.
Last edited by Ken Nelson on Thu 18 Mar, 2010 7:25 pm; edited 1 time in total
| Ken Nelson wrote: |
| I am sorry, but steel does not attain carbon that way. Even in a reducing fire in a forge, the steel is getting hit with a lot of CO2. both O2 and CO2 will pull carbon from the steel, not to mention that if you had the piece in the fire for 5 minutes, the layer of scale that will form as soon as you bring it out of the fire is just as thick, thereby removing any possible added carbon.
I know that "only a coal/charcoal fire can add carbon" is a long standing blacksmith tradition, but it has been tested and found to be a myth. Several hundred samples were treated in forges, and then cut apart and inspected with a microscope. they were all found to have varying levels of carbon loss on the edges. Some were more severe than others, but to add carbon to steel, you need to seal it in an environment without oxygen. that means either using a case carburizing compound which seals the surface or to pack it in carbon containing material, seal it in a tube and then roast the tube for several hours. Carbon only moves through steel at a rate measured in microns per minute. Scale forms thicker and quicker. |
Yeah, but that's during regular forging temperatures. At those temperatures, to get any significant carbon absorption it needs to be in the fire for at least hours. If you get above the burning point, it's a whole different matter. If you get too high, you end up with a puddle of cast iron at the bottom of your forge.
It depends of course how good your fire management is. If your fire management skill is good, there will be a good reducing environment in your forge. If it's poor, by adding far too much air into the forge, you'll still have an oxidizing environment, and you will just burn away the steel inside the forge. And if you use a gass forge it's a whole different matter, as they are far more oxidizing than charcoal forges.
| Quote: |
| I am glad to hear that your seax turned out well, welding an edge steel on is much more difficult than just forging out a straight bar. What steel did you use for the edge? If you used something like W1 or O6 with well over 1.00% carbon, you could lose quite a bit and still get a fully hardenable steel. 1095 is good for that too. |
It was a piece of antique spring steel, welded onto wrought iron.
| Jeroen Zuiderwijk wrote: |
| And if you use a gass forge it's a whole different matter, as they are far more oxidizing than charcoal forges.
|
There is some room for debate on gas forges being inferior to coal. Their design and venturi or blower air mix and volume versus openings has some empirical learning required to make them “neutral.” I have a thermocouple and some predetermined settings for gas pressure and choke that seem to work. (I am working straight stock while others are still working on building up their coal fire.) Many of us use flux when going up to welding heat, and accept scale that tends to form before borax melts (around 400, although 25% of total flux mix of orthoborax "roach kill" powder can melt and protect at the lower temperature range where scale starts), and other losses. The trade off is time saved on fire management. If the finished knife hardens close to manufacturer’s heat treat specifications, I figure it turned out o.k. All of that said, I like the coal and hand cranked blower better for forging, and the gas for welding.
I recommend “Metallurgy of Steel for Bladesmiths & Others who Heat Treat and Forge Steel”, by John Verhoeven, 2005. I was able to download it for free. It is technical, but about as readable as could be possible for this kind of material. And, it is pretty much targeted perfectly for the types of participants of this forum who are seriously working at forging and heat treating blades.
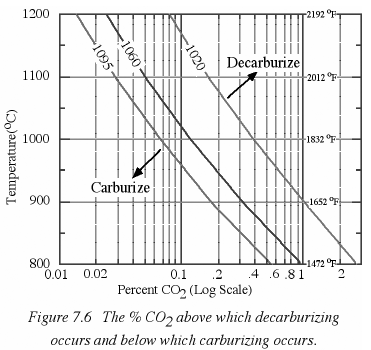
Chapter 7 addresses the reducing versus oxidizing environment and some of the chemical equilibrium effects of CO2.
Page 1 of 1
You cannot post new topics in this forumYou cannot reply to topics in this forum
You cannot edit your posts in this forum
You cannot delete your posts in this forum
You cannot vote in polls in this forum
You cannot attach files in this forum
You can download files in this forum
All contents © Copyright 2003-2006 myArmoury.com — All rights reserved
Discussion forums powered by phpBB © The phpBB Group
Switch to the Full-featured Version of the forum
Discussion forums powered by phpBB © The phpBB Group
Switch to the Full-featured Version of the forum